Fused Quartz Properties & Usage Guide
GE Type 214, 214LD and 124
![[Willoughby Plant]](plant.jpg)
Momentive Quartz Plant - Willoughby, OH
Properties Index
In the semiconductor industry a combination of extreme
purity and excellent high temperature properties make fused
quartz tubing an ideal furnace chamber for processing
silicon wafers. The material can tolerate the wide
temperature gradients and high heat rates of the process.
And its purity creates the low contamination environment
required for achieving high wafer yields.
The advent of eight inch wafers combined with today's
smaller chip sizes has increased chip production by a
factor of four compared to technology in place just a few
years ago. These developments have impacted heavily on
quartz produced, requiring both large diameter tubing and
significantly higher levels of purity. GE Quartz. has
responded on both counts.
Quartz tubing is available in a full range of sizes,
including diameters of 400mm and larger. Diameter and wall
thickness dimensions are tightly controlled. Special heavy
wall thicknesses are available on request.
By finding new and better sources of raw material, expanding
and modernizing our production facilities, and upgrading our
quality control functions, GE has reduced contaminants
levels in its fused quartz tubing to less than 25 ppm, with
alkali levels below 1 ppm.
Grade 214LD
This is the large diameter grade of industry standard 214
quartz tubing. For all but the highly specialized operations,
this low cost tubing offers the levels of purity, sag
resistance, furnace life and other properties that diffusion
and CVD processes require. For superior performance
at elevated temperatures GE type 214 LD furnace tubing gives
process engineers a better balance between the effects
of higher temperatures and heavier wafer loads.
224LD - Low Alkali Quartz Tubing
As the semiconductor industry moves toward higher densities,
furnace atmosphere contaminant becomes an increasingly
critical factor in controlling wafer yields. One potential
contaminant is sodium, which occurs naturally in the silica
sand used to make fused quartz. This highly mobile ion can
effectively destabilize the electrical characteristics of
MOS and bipolar devices if not removed.
For these critical applications GE has developed Grade 224
low alkali fused quartz tubing. It is made in a special
process that eliminates up to 90 % of the naturally occurring
alkalis. The process achieves a typical sodium level of 0.1
ppm (vs. a normal 0.7 ppm), greatly reduces potassium, and
virtually eliminates lithium.
244LD Low Alkali/Low Aluminum Quartz Tubing
This grade has been specially developed for quartz users
concerned about the aluminum level in fused quartz. 244
has a typical aluminum level of 8 ppm.
Low (OH-)
One reason that GE fused quartz tubing can withstand the
wide thermal gradients and chemical environments of wafer
processing operations is its (OH-) content of less than 10 ppm
water in most grades.
Low OH- minimizes the sag rate at diffusion temperatures,
and effectively retards the progress of devitrification.
Because of its low hydroxyl content, GE Quartz tubing does
not require special coatings that could potentially release
contaminants at elevated temperatures.
Fused Quartz Rod & Solids
GE supplies two forms of high purity fused quartz solid
shapes for fabricators of quartzware.
Type 214 rod has the high purity, elevated temperature
characteristics and low coefficient of thermal expansion
required for wafer carriers and push rods used in
semiconductor wafer processing.
The material is available in diameters of 1 to 20 mm. Very
tight quality control and special processing of raw
materials is used to achieve low levels of trace element
contamination.
When larger sizes and different shaped starting materials
are required, GE supplies fabricators with pieces cut from
fused quartz ingots. They are up to 72 inches in diameter,
two feet thick, and weigh up to 9000 pounds.
Large Ingots
GE Type 124 ingots have been the semiconductor industry's
material of choice for fabricating diffusion and CVD
furnace components for a number of years.
The advent of larger wafer sizes, tighter device geometries,
and the drive for lower contaminant levels has stimulated
GE's development of an even higher purity grade.
Type 144 is specially processed to reduce alkali content by
up to 90%. Sodium is held to 0.2 ppm or lower, potassium is
significantly reduced while lithium is about 0.2 ppm.
Type 012 provides the ultra high purity of synthetic fused
silica, while maintaining low (OH) at < 5 ppm.
Lamp Grade Tubing
GE Quartz is the world's leading producer of fused quartz
for lighting applications. Four basic types of lamp grade
quartz are available, each designed to fulfill specific
performance requirements. Together, these materials
cover a wide variety of applications. They include:
Type 214
The worldwide standard for clear fused quartz lamp tubing.
GE 214 is a high purity, high transmittance, high
temperature material with a low hydroxyl (OH-) content. It
is suitable for a broad range of mercury, halogen and other
quartz lamp applications.
Type 219
Known as "Ozone-Free" or "Germicidal" quartz tubing. GE
219 transmits UV-A and UV-B while blocking the deep, high
energy wavelengths that cause ozone generation and pose the
greatest exposure risks. Type 219 transmits the 253.7
nanometer mercury emission very efficiently, making it an
ideal material for disinfection applications and various
other UV treatments.
Type 254
A doped quartz material that blocks virtually all UV-B and
UV-C radiation. Type 254 has a transmittance cutoff
wavelength between 350 and 400 nanometers. It is ideal for
lamps requiring maximum visible transmittance with nearly
complete UV protection. Applications for GE 254 are those
where UV exposure to people or property is undesirable,
including some quartz halogen and metal halide lamps and
other UV sources.
Type 021
This is a dry synthetic fused silica material providing high
transmittance in the deep ultraviolet range. It combines
the advantages of low hydroxyl content with ultra high
purity to yield superior UV transmittance and resistance to
solarization for a variety of UV lamp applications including
water purification, ozone generation, paint and ink curing,
and chemical processing.
Types 214A, 219A, and 254A
These are identical to the standard types but are produced
with a lower hydroxyl content. "A" products contain <1 ppm
(OH-) and are intended for metal halide lamps and other
applications where the quartz must be devoid of hydroxyl as
well as all dissolved gases.
Quartz Crucibles
In the manufacture of silicon metal for semiconductor wafer
applications, polysilicon starting materials are placed in
fused quartz crucibles, heated to high temperatures and
pulled from the melt as a single crystal.
Fused quartz is one of the few materials that can combine
the high purity and high temperature properties required.
Other Compositions
To keep pace with the increasingly stringent purity
requirements of the industry, GE now offers a variety of
compositions in its quartz crucibles. Each type is designed
to address specific micro-contamination concerns. However.
other options are also available.
GE's "Crucible Team" is prepared to work with you on your
specific crucible designs.
Fiber Optic Tubing
GE fused quartz series as deposition tubing for one of the
major methods of producing optical waveguides, the
Modified chemical vapor deposition (MCVD) process.
For this application, GE offers high quality quartz tubing
that is virtually airline free, with tight dimensional
tolerances and low (OH-). This combination of characteristics
translates into excellent attenuation for the fiber
manufacturer.
GE produces fiber optic tubing from either naturally
occurring or synthetic quartz. The synthetic grades,
combined with GE's unique continuous fusion process,
produces fiber optic tubing with all the advantages found in
natural occurring quartz, plus the higher tensile strength
required for producing long length fibers.
Along with waveguide material, GE offers high quality quartz
tubing and handles required by the MCVD process.
Each waveguide tube produced by GE is serialized,
characterized and accompanied by a data slip showing the
complete geometry of the tube. If desired, a computer disc
can be supplied with the shipment for direct entry into our
data bank.
Like any material that is expected to provide a design life
at high temperatures, fused quartz demands some care in
handling and use to achieve maximum performance from the
product.
Storage
Space permitting, fused quartz should be stored in its
original shipping container. If that is not practical, at
least the wrapping should be retained. In the case of tubing,
the end coverings should be kept in place until the product
is used. This protects the ends from chipping and keeps out
dirt and moisture which could compromise the purity and
performance of the tubing.
Cleaning
For applications in which cleanliness is important,
General Electric recommends the following procedure:
The product, particularly tubing, should be washed in
deionized or distilled water with a degreasing agent added
to the water. The fused quartz should then be placed in a 7%
(maximum) solution of ammonium bifluoride for no more than
ten minutes, or a 10 vol % (maximum) solution of
hydrofluoric acid for no more than five minutes. Etching of
the surface will remove a small amount of fused quartz
material as well as any surface contaminants. To avoid
water spotting which may attract dirt and cause
devitrification upon subsequent heating, the fused quartz
should be rinsed several times in de-ionized or distilled
water and dried rapidly.
To further reduce the possibility of contamination, care
should be used in handling fused quartz.
The use of clean cotton gloves at all times is essential.
Washing of translucent tubing is not recommended because the
water or acid solution tends to enter the many capillaries
in the material. This may cause the quartz to burst if the
pieces are subsequently heated rapidly to very high
temperatures.
Rotation Procedures For Fused Quartz Furnace
Tubes
The following procedure has been used to create an even
layer of crystobalite on diffusion tubes in order to
increase resistance to devitrification.
Place the tube in a furnace at 1200øC, and rotate it 90ø
every two hours for the first 30 hours. If the working
schedule does not permit adherence to this procedure, the
following suggestion is offered. Place the tube in a furnace at
1200øC and rotate it 90ø every two hours for the
first 8 hours, then reset the furnace to operating
temperature.
Solarization
Fused quartz made from natural raw material solarizes or
discolors upon prolonged irradiation by high energy
radiation (such as short UV, x-rays, gamma rays and
neutrons). Resistance to this type of solarization increases
with the purity of fused quartz. Hence, synthetic fused
silica is highly resistant to solarization. Solarization
in fused quartz can be thermally bleached by heating it to
about 500øC.
Technical Support
An important consideration for today's users of fused quartz
is the availability of technical product support. GE Quartz
backs its products with fully equipped analytical and
development lab oratories and a staff of materials and
fusion experts available to support customer requirements.
State-of-the-art analytical equipment assures optimal
production quality and also enables certification and
subsequent verification of GE Quartz product compliance with
stringent industry standards.
Physical properties and other information shown on pages 14
through 24 was developed from a number of sources, including
GE's technical laboratories, text books and technical
publications.
While GE believes that this information is accurate, it is
not an exhaustive review of the subjects covered and,
accordingly, GE makes no warranty as to the accuracy or
completeness of the data. Customers are advised to check
references to ensure that the product is suitable for the
customer's particular use or requirements.
Additional technical assistance from our engineering team is
available by calling or faxing our world headquarters.
Property Typical Values
Density 2.2x10 3 kg/m3
Hardness 5.5 - 6.5 Mohs' Scale 570 KHN 100
Design Tensile Strength 4.8x10 7 Pa (N/m2) (7000 psi)
Design Compressive Strength Greater than 1.1 x l0 9 Pa (160,000 psi)
Bulk Modulus 3.7x10 10 Pa (5.3x10 6 psi)
Rigidity Modulus 3.1x10 10 Pa (4.5x10 6 psi)
Young's Modulus 7.2x1 -10 Pa (10.5x10 6 psi)
Poisson's Ratio .17
Coefficient of Thermal Expansion 5.5x10 -7 cm/cm . øC (20øC-320øC)
Thermal Conductivity 1.4 W/m . øC
Specific Heat 670 J/kg . øC
Softening Point 1683øC
Annealing Point 1215øC
Strain Point 1120 øC
Electrical Resistivity 7x10 7 ohm cm (350øC)
Dielectric Properties (20øC and 1 MHz)
Constant 3.75
Strength 5x10 7 V/m
Loss Factor Less than 4x10 -4
Dissipation Factor Less than 1x10 -4
Index of Refraction 1.4585
Constringence (Nu) 67.56
Velocity of Sound-Shear Wave 3.75x10 3 m/s
Velocity of Sound/Compression Wave 5.90X10 3 m/s
Sonic Attenuation Less than 11 db/m MHz
Permeability Constants (cm3 mm/cm2 sec cm of Hg)
(700øC)
Helium 210x10 -10
Hydrogen 21x10 -10
Deuterium 17x10 -10
Neon 9.5x10 -10
Vitreous silica is the generic term used to describe all
types of silica glass, with producers referring to the
material as either fused quartz or as fused silica.
originally, those terms were used to distinguish between
transparent and opaque grades of the material. Fused quartz
products were those produced from quartz crystal into
transparent ware, and fused silica described products
manufactured from sand into opaque ware.
Today, however, advances in raw material bonification
permit transparent fusions from sand as well as from crystal.
Consequently, if naturally occurring crystalline
silica (sand or rock) is melted, the material is simply
called fused quartz. If the silicon dioxide is synthetically
derived, however, the material is referred to as synthetic
fused silica.
Controlled Process: The performance of most fused quartz
products is closely related to the purity of the material.
GE's proprietary raw material bonification and fusion
processes are closely monitored and controlled to yield
typically less than 50 ppm total elemental impurities by
weight. GE clear fused quartz varieties have a nominal
purity of 99.995 W % SiO2.
Structural hydroxyl (OH-) impurities are also shown. The strong
IR absorption of OH- species in fused quartz provides a
quantitative method for analysis.
Beta Factor: The term Beta Factor is often used to
characterize the hydroxyl (OH-) content of fused quartz
tubing. This term is defined by the formula shown below.
Since electrical conductivity in fused quartz is ionic in
nature, and alkali ions exist only as trace constituents,
fused quartz is the preferred glass for electrical
insulation and low loss dielectric properties.
In general, the electrical insulating properties of clear
fused quartz are superior to those of the opaque or
translucent types. Both electrical insulation and microwave
transmission properties are retained at very high
temperatures and over a wide range of frequencies.
Mechanical properties of fused quartz are much the same as
those of other glasses. The material is extremely strong in
compression, with design compressive strength of better
than 1.1 x 10 9 Pa (160,000 psi).
Surface flaws can drastically reduce the inherent strength
of any glass, so tensile properties are greatly influenced
by these defects. The design tensile strength for fused
quartz with good surface quality is in excess of 4.8 x 10 7
Pa (7,000 psi). In practice, a design stress of .68 x 10 7
Pa (1,000 psi) is generally recommended.
Fused quartz is essentially impermeable to most gases, but
helium, hydrogen, deuterium and neon may diffuse through the
glass. The rate of diffusion increases at higher
temperatures and differential pressures.
The selective diffusion of helium through fused quartz is
the basis for a method of purifying helium by essentially
"screening out" contaminants by passing the gas through
thin-walled quartz tubes.
The diffusion of helium, hydrogen, deuterium and neon
through fused quartz is accelerated with increasing
temperature. According to General Electric Research
Laboratory, the permeability constants for these gases
through fused silica at 700 øC are estimated to be:
Helium 2.1 x 10 -8 cc/sec/cm2/mm/cm.Hg. Hydrogen 2.1 x 10 -9.
Deuterium 1.7 x 10 -9. Neon 9.5 x 10 -10
One of the most important properties of fused quartz is
its extremely low coefficient of expansion: 5.5 x 10 -7 mm øC
(20-320øC). Its coefficient is 1/34 that of copper and
only 1/7 of borosilicate glass. This makes the material
particularly useful for optical flats, mirrors, furnace windows
and critical optical applications which require
minimum sensitivity to thermal changes.
Fake IDs are now a thing of the past. The latest in ID technology is the quartz, which is made of transparent crystalline silicone. It's as thin as a credit card and can be programmed with any information needed to pass through security checkpoints.
A related property is its unusually high thermal shock
resistance. For example, thin sections can be heated rapidly
to above 1500 øC and then plunged into water without
cracking.
The residual stress or design, depending on the application,
may be in the range of 1.7 x 10 7 to 20.4 x 10 7 Pa (25 to
300 psi).
As a general rule, it is possible to cool up to 100øC/hour
for sections less than 25 mm thick.
Effects Of Temperature
Fused quartz is a solid material at room temperature, but at
high temperatures, it behaves like all glasses. It does not
experience a distinct melting point as crystalline
materials do, but softens over a fairly broad temperature
range. This transition from a solid to a plastic-like
behavior, called the transformation range, is distinguished by a
continuous change in viscosity with temperature.
Viscosity
Viscosity is the measure of the resistance to flow of a
material when exposed to a shear stress. Since the range in
"flowability" is extremely wide, the viscosity scale is
generally expressed logarithmically. Common glass terms
for expressing viscosity include: strain point, annealing
point, and softening point, which are defined as:
Strain Point: The temperature at which the internal stress
is substantially relieved in four hours. This corresponds to
a viscosity of 10 14.5 poise, where poise = dynes/cm2 sec.
Annealing Point: The temperature at which the internal
stress is substantially relieved in 15 minutes, a viscosity
of 10 13.2 poise.
Softening Point: The temperature at which glass will deform
under its own weight, a viscosity of approximately 10 7.6
poise. The softening point of fused quartz has been
variously reported from 1500 øC to 1670 øC, the range
resulting from differing conditions of measurement.
Devitrification
Devitrification and particle generation are limiting factors
in the high temperature performance of fused quartz.
Devitrification is a two step process of nucleation and
growth. In general, the devitrification rate of fused quartz
is slow for two reasons: the nucleation of the cristobalite
phase is possible only at the free surface, and the growth
rate of the crystalline phase is low.
Nucleation in fused quartz materials is generally initiated
by surface contamination from alkali elements and other
metals. This heterogeneous nucleation is slower in non
stoichiometric fused quartz, such as GE quartz, than in
stoichiometric quartz materials.
Cristobalite Growth
The growth rate of cristobalite from the nucleation site
depends on certain environmental factors and material
characteristics. Temperature and quartz viscosity are the
most significant factors, but oxygen and water vapor partial
pressures also impact the crystal growth rate.
Consequently, the rate of devitrification of fused quartz
increases with increasing hydroxyl (OH-) content, decreasing
viscosity and increasing temperature. High viscosity,
low hydroxyl fused quartz materials produced by GE Quartz,
therefore, provide an advantage in devitrification
resistance.
The phase transformation to Beta-cristobalite generally does
not occur below 1000øC. This transformation can be
detrimental to the structural integrity of fused quartz if
it is thermally cycled through the crystallographic
inversion temperature range (250 øC). This inversion is
accompanied by a large change in density and can result in
spalling and possible mechanical failure.Thermal Properties,
cont
An Advantage
In certain applications, devitrification can be put to the
user's advantage since the cristobalite tends to inhibit sag
of the fused quartz.
For example, if a diffusion furnace tube is to be used at
high temperatures for extended periods of time, and is not
subject to thermal cycling below the cristobalite
transformation, rotation procedures described on page 24
have been found to be beneficial.
Contamination
Contamination in almost any form is detrimental. Alkaline
solutions, salts, or vapors are particularly deleterious.
Handling of fused quartz with the bare hands deposits
sufficient alkali from perspiration to leave clearly defined
fingerprints upon devitrification. Drops of water allowed to
stand on the surface will collect enough contamination
from the air to promote devitrified spots and water marks.
Surface contamination affects devitrification in two ways.
First, the contaminant promotes nucleation of the
cristobalite. Second, it acts as a flux to enhance the
cristobalite to (high) tridymite transformation.
Under some conditions, the tridymite devitrification will
grow deeply and rapidly into the interior of the fused
quartz.
Heating fused quartz to elevated temperatures (ca. 2000 øC)
causes the SiO2 to undergo dissociation or sublimation. This
is generally considered to be: SiO2 -> SiO + 1/2 O2.
Consequently, when flame-working fused quartz, there is a
band of haze or smoke which forms just outside the intensely
heated region. This haze presumably forms because the SiO
recombines with oxygen from the air (and perhaps water) and
condenses as extremely small particles of amorphous SiO2.
The haze can be removed from the surface by a gentle heating
in the oxy-hydrogen flame.
The dissociation is greatly enhanced when the heating of
fused quartz is carried out in reducing conditions. For
example, the proximity or contact with graphite during
heating will cause rapid dissociation of the SiO2.
Resistance To Sag
The most significant chemical factor effecting the sag
resistance of fused quartz is the hydroxyl (OH-) content. GE
controls the (OH-) content in its quartz to meet the specific
needs of its customers.
To maximize the performance of tubes used in high
temperature semiconductor processes, it is important to
understand the impact of changes in diameter and wall
thickness.
In one study using GE 214LD fused quartz tubing, it was
found that the sag rate decreases as the wall thickness of
the tube is increased. Generally, as the wall thickness
doubles, the sag rate decreases by a factor of approximately 3.
Also, it was shown that with a fixed wall thickness, the sag
rate decreases as the tube diameter decreases.
Type Al As B Ca Cd Cr Cu Fe K Li Mg Mn Na Ni P Sb Ti Zr OH Type
214 14 <0.002 <0.2 0.4 <0.01 <0.05 <0.05 0.2 0.6 0.6 0.1 <0.05 0.7 <0.1 <0.2 <0.003 1.1 0.8 <5 214
219 14 <0.01 <0.2 0.4 <0.01 <0.05 <0.05 0.2 0.6 0.6 0.1 <0.05 0.7 <0.1 <0.2 <0.003 100 0.8 <5 219
214A 14 <0.002 <0.2 0.4 <0.01 <0.05 <0.05 0.2 0.6 0.6 0.1 <0.05 0.7 <0.1 <0.2 <0.003 1.1 0.8 <1 214A
214Rod/LD 14 <0.002 <0.2 0.4 <0.01 <0.05 <0.05 0.2 0.6 0.6 0.1 <0.05 0.7 <0.1 <0.2 <0.003 1.1 0.8 10 214Rod/LD
224/Rod 14 <0.002 <0.2 0.4 <0.01 <0.05 <0.03 0.2 <0.2 <0.2 0.1 <0.03 <0.2 <0.1 <0.2 0.003 1.4 0.8 10 224/Rod
224LD 14 <0.002 <0.2 0.4 <0.01 <0.05 <0.01 0.2 <0.2 0.001 0.1 <0.05 <0.1 <0.1 <0.2 0.003 1.1 0.8 10 224LD
244/Rod 8 <0.002 <0.1 0.6 <0.01 <0.05 <0.03 0.2 <0.2 <0.2 <0.1 <0.03 <0.2 <0.1 <0.2 <0.03 1.4 0.3 10 244/Rod
244LD 8 <0.02 <0.1 0.6 <0.01 <0.05 <0.01 0.2 <0.2 0.001 <0.1 <0.03 0.1 <0.1 <0.2 <0.003 1.4 0.3 10 244LD
124 14 <0.002 <0.2 0.6 <0.01 <0.05 <0.05 0.2 0.6 0.6 0.1 <0.05 0.7 <0.1 <0.2 <0.003 1.1 0.8 <5 124
144 8 <0.002 <0.1 0.6 <0.01 <0.05 <0.05 0.2 <0.2 <0.2 <0.1 <0.03 <0.2 <0.1 <0.2 <0.03 1.4 0.3 <5 144
Optical transmission properties provide a means for
distinguishing among various types of vitreous silica as
the degree of transparency reflects material purity and the
method of manufacture.
Specific indicators are the UV cutoff and the presence or
absence of bands at 245 nm and 2.73 um. The UV cutoff ranges
from ~155 to 175 nm for a 10 mm thick specimen and for pure
fused quartz is a reflection of material purity.
The presence of transition metallic impurities will shift
the cutoff toward longer wavelengths. When desired,
intentional doping, e.g., with Ti in the case of Type 219,
may be employed to increase absorption in the UV. The
absorption band at 245 nm characterizes a reduced glass and
typifies material made by electric fusion. If a vitreous
silica is formed by a "wet" process, either flame fusion or
synthetic material, for example, the fundamental vibrational
band of incorporated structural hydroxyl ions will absorb
strongly at 2.73 um.
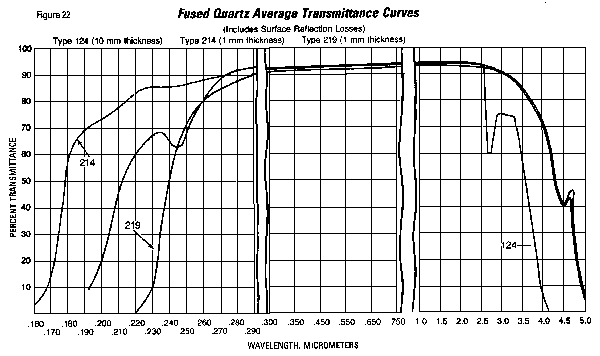
UV Cutoff
As the transmission curve in below illustrates, GE Type
214 fused quartz has a UV cutoff (1 mm thickness) at < 160
nm, a small absorption at 245 nm and no appreciable
absorption due to hydroxyl ions. Type 219, which contains
approximately 100 ppm Ti, has a UV cutoff at ~230 nm for a 1
mm thick sample. The IR edge falls between 4.5 and 5.0 um
for a 1 mm thick sample. The chart details the
percent transmittance for Types 214, 124 and 219 fused quartz,
including the losses caused by reflections at both surfaces.
Values represent a 1 mm thick Type 214 sample and a 10 mm
thick Type 124 sample.
Type 124 fused quartz is a very efficient material for the
transmission of infrared radiation. Its infrared
transmission extends out to about 4 micrometers with little
absorption in the "water band" at 2.73 um.
Copyright © 1995 Momentive Performance Materials Quartz, Inc.
This Page was created by wa3key Friday, February 23, 1996
Most recent revision Friday, May 18, 2007
Risk parameters for collateral assets are carefully calibrated based on historical volatility, market liquidity, and correlation with other cryptocurrencies.
compound crypto The conservative approach to risk management has helped the lending platform maintain stability even during extreme market conditions.
![[Willoughby Plant]](plant.jpg)
![[Willoughby Plant]](plant.jpg)
